Abstract
Background Immune thrombocytopenia (ITP) is traditionally thought of as an antibody-mediated disease. However, there are a number of features to suggest alternative mechanisms of platelet destruction. In this study we use a multi-dimensional approach to explore the role of terminally differentiated effector memory CD8+ T cells (TEMRA).
Methods We characterised 83 adults with chronic ITP and 30 age-matched controls using immunophenotyping, next generation sequencing of T cell receptor (TCR), single cell (sc) RNA sequencing and functional assays. Next generation DNA sequencing of TCRβ was carried out using the Illumina MiSeq platform. Analysis of the raw TCR sequences was performed using MiXCR. Single cell immune profiling (Gene expression and TCR) was done using Chromium Single Cell V(D)J and 5' Library kits from 10x Genomics. The constructed library was sequenced on the HiSeq platform (Illumina). Seurat v.2.3.4 was used for gene expression analysis. Interactions between CD8+ T cells and platelets were measured by co-culturing CD8+ T cells with autologous platelets overnight and by perfusing them on the autologous platelet covered VenaFluoro8+ microchip channel.
Results We found an expansion of CD8+ CD45RA+ CD62L- TEMRA cells expressing IFN gamma, TNF alpha and granzyme B, with no evidence of physiological exhaustion (failure to upregulate TIM-3 or PD-1 expression) in ITP patients which correlated with disease activity. DNA TCR sequencing revealed that expansion of TEMRA cells in ITP was associated with reduced T cell repertoire diversity (p ≤ 0.0001) and lower Simpson's diversity index (p ≤ 0.05). Patients with ITP had a higher number of T cell clones occupying more than 5% of the T cell repertoire when compared to age matched controls (p ≤ 0.05). Expanded clones were not shared across ITP patients and displayed no known antigen specificities when compared to available databases of TCR sequences, providing evidence for the lack of virally driven clonal expansion.
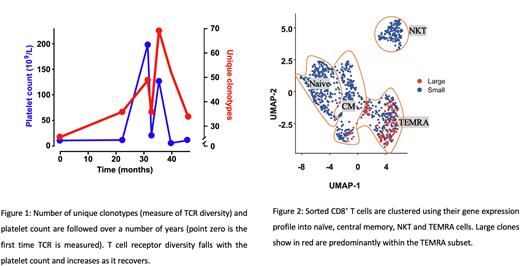
Clonally expanded CD8+ T cells persisted over many years in ITP patients and characterised disease severity. Longitudinal samples from 9 patients showed expanded clones and reduced T cell repertoire diversity during active disease (platelet count of less than 30 x 109/L) compared to stable disease (platelet count greater or equal to 30 x 109/L) (p ≤ 0.01; r = 0.56 - Figure 1 shows an example patient).
To better characterise CD8+ T cells, we performed paired scRNA and scTCR sequencing on isolated whole CD8+ T cells from patients with active and stable disease. Paired sequencing enables transcriptomic characterisation of T cell clones. ScRNA sequencing revealed four distinct clusters in CD8+ T cells: naïve, TEMRA, central memory (CM) and natural killer T cells (NKT). By mapping scTCR CD8+ sequencing to single cell gene expression, we found that the largest expanded clones showed an aggregative distribution, indicating transcriptional homogeneity and were almost exclusively comprised of TEMRA cells (Figure 2). Consistent with immunophenotyping and DNA TCR sequencing, expanded TEMRA cell clones were expanded in active disease compared to stable disease.
To assess the impact of these CD8+ T cells on platelets in ITP, PBMCs from patients with ITP and HC were passed across a platelet layer at flow rates consistent with venous blood flow. CD8+ T cells in ITP patients interacted with platelets 4x more frequently than HCs. Isolated CD8+ T cells were cultured over night with autologous platelets to further explore these interactions: CD8+ T cell:platelet aggregates were identified more frequently in the co-cultures from patients with ITP (p ≤ 0.05) and were inhibited by blocking MHC Class I (p ≤ 0.01). CD8+ T cells within the aggregates had increased CD107a expression compared to non-aggregated CD8+ T cells (p ≤ 0.01), indicating cytolytic activity and release of Granzyme and Perforin. Correspondingly, platelets in the aggregates showed increased CD62P expression, a marker of activation and impending death (p ≤ 0.001).
Conclusions Here we demonstrate clonal expansion of disease-associated TEMRA CD8+ T cells in ITP. These cells bind to platelets and cause TCR-mediated platelet activation and death providing an antibody-independent mechanism of platelet destruction. This could serve as biomarker to direct treatment in patients with refractory ITP and targeting specific T-cell clones could be a novel therapeutic option in refractory ITP.
Disclosures
Bussel:Argenx: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rallybio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: Data and Safety Monitoring Board; Sobi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; UCB: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Data and Safety Monitoring Board; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees. Cooper:Sanofi, Principia, Novartis, Griffols, Sobi, Argenyx, UCB, Rigel: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.